PATHOPHYSIOLOGY OF VOD/SOS
VOD/SOS can progress to multi-organ failure within a few days1
Take a closer look at the cascade of events that precedes the clinical manifestations of VOD/SOS.
Progressive cascade of VOD/SOS
Based on experimental models, buildup of toxic metabolites from HSCT conditioning regimens may trigger activation of and damage to sinusoidal endothelial cells in the liver. This can lead to a cascade of events that is potentially life-threatening.2-5
Hepatocytes
Endothelial cells
Red blood cells
White blood cells
Platelets
Extracellular matrix
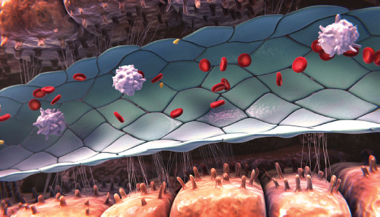
Healthy sinusoid
- Sinusoidal endothelial cells provide a barrier between the blood and hepatocytes and regulate hemostasis, permeability, vascular tone, and immune and inflammatory responses6

Endothelial damage
- Activation of endothelial cells triggers expression of cytokines and adhesion molecules, activating inflammatory pathways that cause additional endothelial damage4,5
- Release of the enzyme heparanase contributes to degradation of the extracellular matrix, loss of cytoskeletal structure, and gap formation between sinusoidal endothelial cells3,5
- Degradation of the extracellular matrix leads to detachment of endothelial cells from the sinusoidal lining6,7
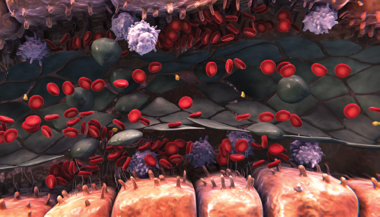
Sinusoidal narrowing
- This ongoing degradation of the endothelium allows red blood cells, leukocytes, and cellular debris to move into the space of Disse3-5
- This can lead to sinusoidal narrowing, which may lead to endothelial cells embolizing downstream and contribute to sinusoidal blockage3-5
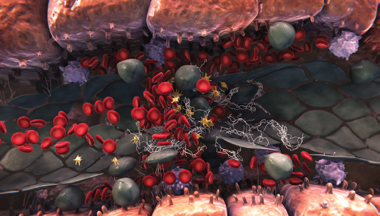
Sinusoidal blockage
- Sinusoidal endothelial cell damage also triggers the expression of multiple factors that regulate coagulation and fibrinolysis (table A)2,4,5
- All of these events can lead to a procoagulant and hypofibrinolytic state, where fibrin deposition, clot formation, and sinusoidal narrowing can cause further blockage2,4,5
- Hepatocyte cell death is a consequence of sinusoidal disruption, and further sinusoidal obstruction may lead to a reduction in hepatic venous outflow and to postsinusoidal hypertension3-6
The cascade of events appears to start before clear clinical and LABORATORY manifestations are evident.4,5,8
TABLE A: FACTORS THAT REGULATE COAGULATION AND FIBRINOLYSIS5,9,10
Clinical manifestations and progression of VOD/SOS
Sinusoidal obstruction may lead to a reduction in hepatic venous outflow and to postsinusoidal hypertension, resulting in clinical signs and symptoms.3-5
Most common signs and symptoms of VOD/SOS3,8,11
- Excessive platelet transfusions consistent with refractory thrombocytopenia
- Weight gain due to fluid retention
- Hepatomegaly
- Right upper quadrant pain
- Elevated bilirubin
- Elevated serum transaminase and alkaline phosphatase
- Ascites
Vigilant monitoring for the first 21 days after hsct is critical to detect VOD/SOS.11
Although VOD/SOS generally emerges within the first 21 days, it can occur later.11,12
Recognize the factors shown to greatly increase the risk of VOD/SOS in patients post HSCT8,12-15
Level of Riska
Patient and Disease-
Related Risk Factors
HEPATIC-RELATED
RISK FACTORS
TRANSPLANT-RELATED
RISK FACTORS
10-20 times
greater risk
- Prior norethindrone
treatment
- Previous inotuzumab treatment
- Previous gemtuzumab treatment
- Bilirubin >1.5 mg/dL
3-10 times
greater risk
- GSTM1 null genotype
- Sepsis post HSCT
- Age (age <2 years, older age)
- Previous liver disease
- Ferritin ≥950 ng/mL
- Elevated AST/ALTb
- High intensity/MAC regimens
- GvHD prophylaxis
- Horse ATG treatment
(aplastic anemia)
1-3 times
greater risk
- Inborn errors of metabolism
- Leukemia diagnosis
- Previous abdominal radiation
- Impaired pulmonary function
- KPS score <90%
- ECOG PS 2-4
- Advanced disease status
- Elevated AST/ALT
- Hepatitis C
- Previous HSCT
- Allogeneic vs autologous HSCT
- Unrelated/HLA mismatch
- Total body irradiation
- Early neutrophil engraftment
aBased on odds ratio. Odds ratio represents the odds that a complication will occur if the risk factor is present, compared with the odds of the complication occurring if the risk factor is absent.15
bThe odds ratio for elevated AST/ALT ranges from 2.4 to 4.6.14
Previous use of inotuzumab and gemtuzomab carries increased risk for VOD/SOS
Incidence of VOD/SOS post HSCT following prior inotuzumab ozogamicin treatment16,17
Incidence of VOD/SOS post HSCT following prior gemtuzumab ozogamicin treatment18
cIn a study of 58 patients (including 1 adolescent) with R/R B-ALL to evaluate treatment with inotuzumab ozogamicin prior to allo-HSCT between 2016 and 2022. Seventeen of 56 patients had VOD/SOS, and 9 of those patients (53%) died due to VOD/SOS with multi-organ failure.16
dIn a Phase 3 multicenter, open‐label, randomized study that included 326 patients (inotuzumab ozogamicin [n=164] or investigator's choice of chemotherapy [n=162]) with R/R ALL, 79 of whom received inotuzumab ozogamicin and subsequent allogeneic HSCT. Five of the 18 VOD/SOS events that occurred post HSCT were fatal. Of the 32 patients in the standard care group (n=143) who underwent follow-up HSCT, 1 case of VOD/SOS was reported and was fatal.17,19
More recent treatment options carry their own risks of VOD/SOS
CAR T20
Retrospective, multicenter study of 39 adult patients who underwent an allo-HSCT after anti-CD19 CAR T therapy between January 2017 and April 2021 for LBCL. It demonstrated that this CAR T therapy may pose a high risk for hepatic toxicity and VOD
Risk factors in patients with hepatic VOD:
Conditioning intensitye:
- Low: n=4
- Intermediate: n=2
Interim anticancer therapy:
- Received 1 therapy: n=2
- Received 2 therapies: n=1
Gene therapy21
Phase 3 trial of 52 patients with transfusion-dependent β-thalassemia who received gene therapy after preparing with busulfan conditioning, which poses a known risk of VOD
Hepatic VOD was the most common serious AE, occurring in 5 of 52 patients, and was attributed to busulfan conditioning
eLow-intensity conditioning was thiotepa-based and intermediate-intensity conditioning included fludarabine and treosulfan.20
Myeloablative conditioning Regimens ALSO heighten the risk of VOD22
An open-label, randomized, placebo-controlled study of patients undergoing myeloablative HSCT found VOD incidence of 12% (n=22/180)